Polycystic kidney disease (PKD) isn’t just about fluid-filled sacs in the kidneys. It’s a genetic time bomb that slowly destroys kidney function over decades-often without warning. Around 600,000 people in the U.S. live with it, and half of them will need dialysis or a transplant by age 60. What makes PKD so dangerous isn’t just the cysts-it’s how quietly it progresses, how hard it is to diagnose early, and how few treatment options still exist. But understanding the genetics and modern management strategies can change the outcome.
How PKD Is Inherited: It’s Not Just Family History
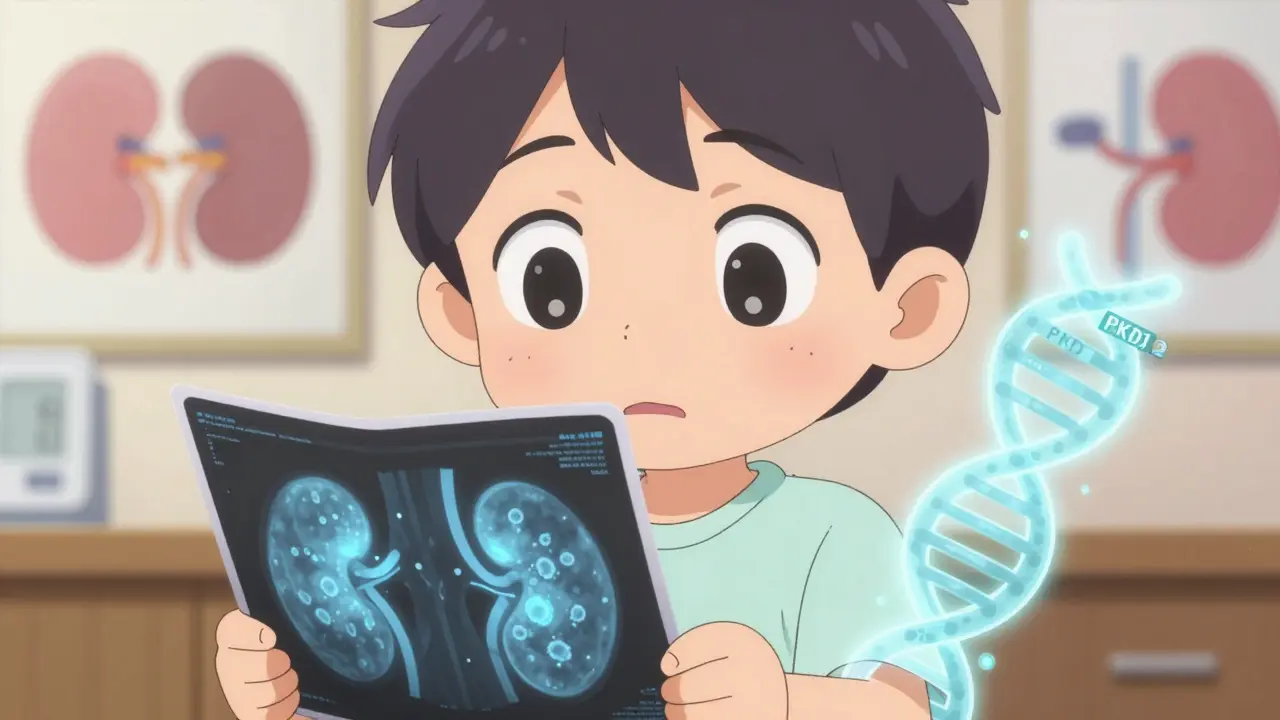
PKD doesn’t skip generations. It’s passed down through specific genes. There are two main types: autosomal dominant (ADPKD) and autosomal recessive (ARPKD). ADPKD makes up more than 98% of cases. If one parent has it, each child has a 50% chance of getting it. That’s not a small risk-it’s a coin flip. The gene mutations involved are either PKD1 or PKD2. PKD1 is the bigger problem: it causes more cysts, faster growth, and kidney failure about 20 years earlier than PKD2.
But here’s the twist: 10% of ADPKD cases happen with no family history. That means someone’s child could be the first in the family to have it. These are called de novo mutations. They’re random, not inherited. That’s why a person with no known relatives with kidney disease can still be diagnosed with PKD in their 30s.
ARPKD is different. It’s rare-about 1 in 20,000 births. Both parents must carry a mutated PKHD1 gene, even if they’re perfectly healthy. Each child has a 25% chance of having ARPKD, a 50% chance of being a silent carrier, and a 25% chance of being completely unaffected. This type often shows up in newborns with enlarged kidneys and breathing problems. Some babies don’t survive the first month. Others live into childhood, but with severe liver and kidney damage.
What Happens Inside the Kidneys
Your kidneys normally filter waste and balance fluids. In PKD, the cells lining the kidney tubules start growing abnormally. Instead of forming tight tubes, they create fluid-filled cysts. These cysts grow like balloons-slowly, steadily, and relentlessly. By age 40, someone with ADPKD might have hundreds of cysts. By 60, the kidneys can swell to three or four times their normal size and weigh up to 30 pounds each.
These cysts don’t just take up space. They crush healthy tissue. They mess with blood flow. They trigger inflammation. Over time, the kidneys lose their ability to filter. That’s when creatinine rises, eGFR drops, and symptoms like fatigue, swelling, and high blood pressure become impossible to ignore.
What’s worse? The cysts don’t stay in the kidneys. They show up in the liver, pancreas, and even the heart valves. About 80% of people with ADPKD develop liver cysts. Most don’t cause problems, but some get so large they press on other organs, causing pain or infection.
When Do Symptoms Show Up? It Depends on the Type
ADPKD usually doesn’t cause symptoms until you’re in your 30s or 40s. But that doesn’t mean it’s not active. Cysts are growing from childhood. High blood pressure is often the first sign-even in teens. In fact, 60% of ADPKD patients develop hypertension before age 30. That’s why doctors now recommend checking blood pressure annually starting at diagnosis.
Some people have symptoms earlier. A small group of kids with PKD1 mutations develop severe disease before age 10. These cases are rare but serious. They can lead to kidney failure by their teens. Others don’t feel anything until they’re 50. That’s why PKD is called a “time bomb.” You can’t predict when it’ll go off.
ARPKD is the opposite. Symptoms often appear at birth. Babies might have enlarged kidneys visible on ultrasound. They struggle to breathe because their lungs are compressed. Many need breathing support right after delivery. Those who survive infancy face chronic kidney disease and liver scarring (fibrosis) that gets worse over time.

How Doctors Diagnose PKD Today
There’s no single blood test for PKD. Diagnosis relies on imaging and genetics. For ADPKD, if you have a family history and are over 30, finding 10 or more cysts on an ultrasound confirms the diagnosis. If you’re younger, you need more cysts: at least 2 per kidney if you’re under 30. MRI is the most accurate-it shows cyst size and growth rate over time. CT scans work too, but they use radiation, so they’re not ideal for long-term monitoring.
Genetic testing is becoming more common. A blood or saliva sample can check for mutations in PKD1, PKD2, and PKHD1. It costs around $1,200 now, down from $5,000 a decade ago. Insurance often covers it if you have a family history, early symptoms, or are considering having kids. Genetic testing isn’t just for diagnosis-it helps predict how fast the disease might progress. Someone with a PKD1 mutation is likely to need a transplant sooner than someone with PKD2.
For ARPKD, prenatal ultrasound can spot enlarged kidneys as early as 20 weeks. After birth, liver scans are critical because liver damage often happens before kidney failure.
Current Treatments: Slowing the Damage
There’s no cure. But there are ways to slow it down. The most important thing? Controlling blood pressure. Keeping it below 130/80 mmHg cuts kidney damage by nearly half. ACE inhibitors and ARBs are the go-to drugs. They don’t just lower blood pressure-they protect the kidney filters directly.
The only FDA-approved drug that targets PKD itself is tolvaptan (Jynarque). It blocks a hormone called vasopressin that makes cysts grow. In clinical trials, it slowed kidney function decline by 1.3 mL/min per year. That might not sound like much, but over 10 years, that’s the difference between needing dialysis at 55 or 65. The catch? It costs about $115,000 a year. Side effects include extreme thirst, frequent urination, and liver toxicity. It’s only for people with rapidly progressing disease-confirmed by imaging and eGFR decline.
Other drugs are in the pipeline. Lixivaptan, a similar drug, showed promise in phase 3 trials in 2024. Bardoxolone methyl, originally tested for diabetic kidney disease, improved kidney function by nearly 5 mL/min in early PKD trials. These aren’t cures, but they’re steps forward.
What You Can Do Every Day
Medicines help, but lifestyle matters just as much. Here’s what works:
- Drink water-every day. Studies show high fluid intake may slow cyst growth by suppressing vasopressin.
- Limit salt-under 2,300 mg a day. Less salt means lower blood pressure and less kidney stress.
- Avoid NSAIDs like ibuprofen and naproxen. They reduce blood flow to the kidneys and can speed up damage.
- Exercise regularly-even walking 30 minutes a day helps control weight and blood pressure.
- Don’t smoke. Smoking doubles the risk of kidney failure in PKD patients.
Chronic pain is the #1 complaint among PKD patients. Cysts can rupture, bleed, or get infected. Heat pads, physical therapy, and sometimes nerve blocks help. Opioids are avoided unless absolutely necessary-they don’t fix the root problem.

The Long-Term: Dialysis and Transplant
By age 70, 75% of people with ADPKD will need dialysis or a transplant. The good news? Kidney transplants work well for PKD patients. Survival rates are higher than for other causes of kidney failure. A transplanted kidney doesn’t develop cysts-it’s healthy tissue. The old kidneys can be left in place unless they’re huge or infected.
Wait times vary. In some places, it’s 3 years. In others, it’s 5. Blood type matters: O-negative patients wait the longest. Living donor transplants cut wait time dramatically. Family members can be tested to see if they’re compatible.
After transplant, patients take immunosuppressants for life. But many return to full activity-working, traveling, even having children. One patient in a 2023 survey said, “I went from needing dialysis three times a week to running marathons. It wasn’t magic-it was a transplant and sticking to my meds.”
Where Research Is Headed
Scientists are no longer just treating symptoms. They’re targeting the root cause. New drugs in trials aim to:
- Stop cysts from forming by fixing the faulty protein (polycystin-1)
- Block signals that make kidney cells turn into cyst-lining cells
- Use gene editing (CRISPR) to correct mutations in early-stage embryos-still experimental
The HALT-PKD study showed that ultra-tight blood pressure control (below 110/75) slowed kidney growth by 14% over five years. That’s a big win. It proves that aggressive management works.
And the economic picture? PKD costs the U.S. healthcare system $45,000 per patient annually. Before dialysis, that jumps to $95,000. With new drugs and earlier diagnosis, those numbers could drop. That’s why pharmaceutical companies are investing billions.
Living With PKD: Hope Is Real
It’s not easy. Many people feel isolated. One Reddit user wrote, “It took seven years to get diagnosed-even though my dad had it.” That’s not rare. PKD is often mistaken for simple kidney stones or chronic pain.
But early detection changes everything. A patient who started blood pressure meds at 28 kept their kidney function at 65% at age 45. That’s 17 years of preserved function. That’s quality time with family. That’s avoiding dialysis longer.
Support groups like the PKD Foundation offer real advice-not just medical facts. They help you find specialists, navigate insurance, and cope with anxiety. You’re not alone. And science is catching up faster than ever.
Is polycystic kidney disease always inherited?
No. About 90% of autosomal dominant PKD (ADPKD) cases are inherited from a parent, but 10% happen due to new, spontaneous mutations. That means someone can be the first in their family to have it. Autosomal recessive PKD (ARPKD) requires both parents to carry the gene, even if they don’t have symptoms.
Can you have PKD and not know it?
Yes. Many people with ADPKD don’t have symptoms until their 30s or 40s. High blood pressure is often the only early sign. Some people live for years without realizing they have cysts until a scan for another reason reveals them. That’s why family history matters-even if you feel fine.
What’s the difference between PKD1 and PKD2?
PKD1 mutations cause more aggressive disease. People with PKD1 typically develop kidney failure in their 50s, while those with PKD2 often don’t need dialysis until their 70s. PKD1 is also more common, accounting for about 78% of ADPKD cases. The cysts grow faster and are more numerous with PKD1.
Is tolvaptan (Jynarque) right for everyone with PKD?
No. Tolvaptan is only approved for adults with rapidly progressing ADPKD, confirmed by imaging and declining kidney function. It’s expensive, has serious side effects like liver damage and extreme thirst, and requires regular blood tests. It’s not for slow-progressing cases or ARPKD.
Can you prevent PKD if it runs in your family?
You can’t prevent the gene mutation, but you can prevent complications. Regular blood pressure checks, avoiding NSAIDs, staying hydrated, and not smoking can delay kidney damage by decades. Genetic testing and early monitoring give you a head start on managing the disease before it causes harm.
What happens if PKD is left untreated?
Untreated PKD leads to progressive kidney damage. High blood pressure worsens, cysts keep growing, and kidney function declines. Without intervention, most people reach end-stage kidney disease by age 60-70. That means dialysis or transplant becomes necessary. Liver cysts, heart valve issues, and brain aneurysms can also develop without proper monitoring.
11 Comments
Randolph Rickman
Just got my first ultrasound results and found out I’ve got PKD. I’m 34. No family history. Felt fine until my BP hit 150/95 last month. This post saved my life. I’m starting tolvaptan next week and drinking 3 liters of water daily. No more ibuprofen. I’m not giving up.
Josias Ariel Mahlangu
People act like this is a new problem. My grandfather died on dialysis in 1987. They knew about PKD back then. What’s changed? Nothing but the price tag on the drugs. Stop acting like science just woke up.
Andrew Sychev
So let me get this straight. You’re telling me someone can have a genetic time bomb ticking inside them for decades, and the only thing we can do is drink water and take a $115k drug that makes you pee like a fountain? And you call this progress? This isn’t medicine. It’s a ransom note with a stethoscope.
Dan Padgett
It’s funny how we treat the body like a machine you can fix with pills. But PKD? It’s not broken. It’s a story written in DNA, and we’re just reading it late. The cysts aren’t invaders-they’re echoes of a mistake that happened before you were born. We don’t cure it. We learn to live with the echo. And maybe, one day, we’ll learn to rewrite it.
Hadi Santoso
hey all-just wanna say if you're new to this, the pkd foundation has a free mentor program. i got matched with someone who'd had a transplant 12 years ago and she told me to start walking 20 mins a day. no fancy gear, just sneakers. it changed everything. also, drink water like your life depends on it-because it kinda does. and yes, i typoed. sorry.
Arun ana
So true about the blood pressure 🙏 I started checking mine daily after my dad’s diagnosis. Now I’m 38, BP steady at 122/78, eGFR still 72. Small wins. And yeah, I drink water like it’s my job 😅
Kayleigh Campbell
They say tolvaptan slows progression by 1.3 mL/min per year. That’s like saying your car’s gas tank leaks a little slower. Congrats, we’ve turned a flood into a drip. Still gonna need a new engine. And yeah, I’m still mad about the $115k price tag. Who’s paying for my future dialysis? My grandkids?
Kim Hines
I’ve had PKD since I was 22. Never told anyone. Just took the meds. Didn’t talk about it. Now I’m 47. Kidneys still working. No transplant. Just quiet. That’s all I need.
Joanna Ebizie
So you're telling me my 16-year-old kid might get this because I didn't get tested? And now I'm supposed to feel guilty? Great. Thanks for the guilt trip with a side of genetic roulette.
sue spark
I’m 41 and just found out I have PKD2. My dad had PKD1 and was on dialysis by 52. I’m not panicking. I’m drinking water, walking, and seeing a nephrologist next week. I’ve got time. And I’m not letting this define me
Tiffany Machelski
Just read this and cried. My mom had it. She never told me until she was 50. I’m 28. Going for a scan tomorrow. Praying it’s nothing. But if it is… I’m ready. I’ve got this